Disufenton sodium
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
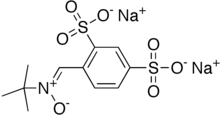
| Formula | C11H13NNa2O7S2 |
| Molar mass | 381.32 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Disufenton sodium (Cerovive, OKN-007, NXY-059, HPN-07)[1] is a free radical trapping nitrone-based antioxidant compound that has been under development for several medical conditions.[2][3]
Chemistry
Disufenton sodium is the disulfonyl derivative of the neuroprotective nitrone spin trap phenylbutylnitrone or "PBN". PBN and its derivatives hydrolyze and oxidize in vitro to form respectively MNP-OH (AKA, NtBHA) and its parent spin-trap MNP.
Research
Disufenton sodium was under development at the drug company AstraZeneca. A 2005 phase-3 clinical trial[4][5] called "SAINT-1" reported some efficacy in the acute treatment of ischemia injury due to stroke. However, a 2006 attempt to repeat this trial indicated no significant activity. After ruling out other causes, the authors tentatively attributed the positive results in the first trial to "chance".[4] AstraZeneca then terminated the development programme.[6]
Disufenton sodium has been researched as a potential treatment for use in brain tumors and cancers, including diffuse intrinsic pontine glioma (DIPG)[7][8] and glioblastoma.[9][10]
A compound (NHPN-1010) containing a combination of disufenton sodium and acetylcysteine has been researched as a potential treatment for tinnitus and hearing loss.[11][12][13][14]
References
- ^ Varela-Nieto I, Murillo-Cuesta S, Rodríguez-de la Rosa L, Oset-Gasque MJ, Marco-Contelles J (September 1, 2021). "Use of Radical Oxygen Species Scavenger Nitrones to Treat Oxidative Stress-Mediated Hearing Loss: State of the Art and Challenges". Frontiers in Cellular Neuroscience. 15. Frontiers Media SA: 711269. doi:10.3389/fncel.2021.711269. PMC 8440819. PMID 34539349.
- ^ Zhao Z, Cheng M, Maples KR, Ma JY, Buchan AM (August 2001). "NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat". Brain Research. 909 (1–2). Elsevier BV: 46–50. doi:10.1016/s0006-8993(01)02618-x. PMID 11478919. S2CID 36633423.
- ^ Choi SH, Choi CH (December 2015). "Noise-Induced Neural Degeneration and Therapeutic Effect of Antioxidant Drugs". Journal of Audiology & Otology. 19 (3). The Korean Audiological Society: 111–119. doi:10.7874/jao.2015.19.3.111. PMC 4704551. PMID 26771008.
- ^ a b Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. (February 2006). "NXY-059 for acute ischemic stroke". The New England Journal of Medicine. 354 (6): 588–600. doi:10.1056/NEJMoa052980. PMID 16467546.
- ^ Lees KR, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, et al. (December 2006). "Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial". Stroke. 37 (12): 2970–2978. doi:10.1161/01.STR.0000249410.91473.44. PMID 17068304.
- ^ "Renovis: Press Release". Archived from the original on October 28, 2006.
- ^ Thomas L, Smith N, Saunders D, Zalles M, Gulej R, Lerner M, et al. (November 2020). "OKlahoma Nitrone-007: novel treatment for diffuse intrinsic pontine glioma". Journal of Translational Medicine. 18 (1). Springer Science and Business Media LLC: 424. doi:10.1186/s12967-020-02593-5. PMC 7654606. PMID 33168005.
- ^ "FDA grants fast track status to OKN-007 for diffuse intrinsic pontine glioma". Healio. March 3, 2021. Retrieved February 5, 2022.
- ^ Battiste JD, Ikeguchi A, Woo S, Sharan S, Zhao YD, Cohoon A, et al. (May 20, 2020). "Phase Ib clinical trial of OKN-007 in recurrent malignant glioma". Journal of Clinical Oncology. 38 (15_suppl). American Society of Clinical Oncology (ASCO): 2538. doi:10.1200/jco.2020.38.15_suppl.2538. ISSN 0732-183X. S2CID 219772612.
- ^ Zalles M, Smith N, Saunders D, Lerner M, Fung KM, Battiste J, Towner RA (January 2022). "A tale of two multi-focal therapies for glioblastoma: An antibody targeting ELTD1 and nitrone-based OKN-007". Journal of Cellular and Molecular Medicine. 26 (2). Wiley: 570–582. doi:10.1111/jcmm.17133. PMC 8743651. PMID 34910361.
- ^ Lu J, West MB, Du X, Cai Q, Ewert DL, Cheng W, et al. (January 7, 2021). Biagini G (ed.). "Electrophysiological assessment and pharmacological treatment of blast-induced tinnitus". PLOS ONE. 16 (1). Public Library of Science (PLoS)\: e0243903. Bibcode:2021PLoSO..1643903L. doi:10.1371/journal.pone.0243903. PMC 7790300. PMID 33411811.
- ^ Zhang J (November 2019). "Blast-induced tinnitus: Animal models". The Journal of the Acoustical Society of America. 146 (5). Acoustical Society of America (ASA): 3811–3831. Bibcode:2019ASAJ..146.3811Z. doi:10.1121/1.5132551. PMID 31795642. S2CID 208621727.
- ^ "Congressionally Directed Medical Research Programs (CDMRP) Search Awards". Congressionally Directed Medical Research Programs. Retrieved February 5, 2022.
- ^ Martin B (July 27, 2020). "Hough Ear Institute receives $300K grant to support research treatments for hearing loss". KOKH. Retrieved February 5, 2022.
Further reading
- Fong JJ, Rhoney DH (March 2006). "NXY-059: review of neuroprotective potential for acute stroke". The Annals of Pharmacotherapy. 40 (3): 461–471. doi:10.1345/aph.1E636. PMID 16507608. S2CID 38016035.
- Warner DS, Sheng H, Batinić-Haberle I (August 2004). "Oxidants, antioxidants and the ischemic brain". The Journal of Experimental Biology. 207 (Pt 18): 3221–3231. doi:10.1242/jeb.01022. PMID 15299043. S2CID 15392635.