Intrathecal_administration
| Subarachnoid space | |
|---|---|
 Diagrammatic representation of a section across the top of the skull, showing the membranes of the brain, etc. ("Subarachnoid cavity" visible at left.) | |
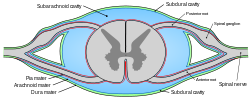 Diagrammatic transverse section of the medulla spinalis and its membranes. (Subarachnoid cavity colored blue.) | |
| Details | |
| Identifiers | |
| Latin | Spatium subarachnoideum, cavum subarachnoideale |
| Anatomical terminology | |
Intrathecal administration is a route of administration for drugs via an injection into the spinal canal, or into the subarachnoid space so that it reaches the cerebrospinal fluid (CSF). It is useful in several applications, such as for spinal anesthesia, chemotherapy, or pain management. This route is also used to introduce drugs that fight certain infections, particularly post-neurosurgical. Typically, the drug is given this way to avoid being stopped by the blood–brain barrier, as it may not be able to pass into the brain when given orally. Drugs given by the intrathecal route often have to be compounded specially by a pharmacist or technician because they cannot contain any preservative or other potentially harmful inactive ingredients that are sometimes found in standard injectable drug preparations.
Intrathecal pseudodelivery is a technique where the drug is encapsulated in a porous capsule that is placed in communication with the cerebrospinal CSF. In this method, the drug is not released into the CSF. Instead, the CSF is in communication with the capsule through its porous walls, allowing the drug to interact with its target within the capsule itself. This allows for localized treatment while avoiding systemic distribution of the drug, potentially reducing side effects and enhancing the therapeutic efficacy for conditions affecting the central nervous system.
The route of administration is sometimes simply referred to as "intrathecal"; however, the term is also an adjective that refers to something occurring in or introduced into the anatomic space or potential space inside a sheath, most commonly the arachnoid membrane of the brain or spinal cord[1] (under which is the subarachnoid space). For example, intrathecal immunoglobulin production is production of antibodies in the spinal cord.[2] The abbreviation "IT" is best not used; instead, "intrathecal" is spelled out to avoid medical mistakes.[citation needed]
Applications of intrathecal administration
Analgesics
Intrathecal administration is often used for a single 24-hour dose of analgesia (opioid with local anesthetic). Caution should be exercised with intrathecal opioids due to the risk of late onset hypoventilation. The use of intrathecal morphine may be limited by severe pruritus and urinary retention.[citation needed]
Pethidine has the unusual property of being both a local anaesthetic and opioid analgesic, which occasionally permits its use as the sole intrathecal anaesthetic agent.[citation needed]
An intrathecal pump system can be used to deliver a local anaesthetic, and/or an opioid and/or an atypical analgesic agent as ziconotide.
Antifungals
Amphotericin B is administered intrathecally to treat fungal infections involving the central nervous system infections.[3]
Cancer chemotherapy
Currently, only four agents are licensed for intrathecal cancer chemotherapy: methotrexate, cytarabine, hydrocortisone, and thiotepa.[4]
Administration of any vinca alkaloids, especially vincristine, via the intrathecal route is nearly always fatal.[5][6][7]
Baclofen
Often reserved for spastic cerebral palsy, baclofen can be administered through an intrathecal pump implanted just below the skin of the abdomen or behind the chest wall, with a catheter connected directly to the base of the spine. Intrathecal baclofen pumps sometimes carry serious clinical risks, such as infection or a possibly fatal sudden malfunction.[citation needed]
Mesenchymal Stem Cell Therapy
Treatment of chronic spinal injuries via the administration of mesenchymal stem cells,[8] either from adipose tissue or bone marrow, is experimental, with better results from the former method. Introduction of mesenchymal stem cells promote the microenvironment needed for axonal regrowth and reduction of inflammation caused by astrocytes proliferation and glial scar tissue.[9]
Animal models have showed improved motor control under the site of injury. A clinical trial also showed statistically significant improved sensitivity under the site of injury in patients.[10]
See also
- Cancer pain/Interventional/Intrathecal pump
- History of neuraxial anesthesia
- Intrathecal pump
- Theca
- Thecal sac
References
- ^ "Route of Administration". Data Standards Manual. Food and Drug Administration. Retrieved 11 March 2011.
- ^ Meinl, E; Krumbholz, M; Derfuss, T; Junker, A; Hohlfeld, R (2008). "Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis". Journal of the Neurological Sciences. 274 (1–2): 42–4. doi:10.1016/j.jns.2008.06.032. PMID 18715571. S2CID 34995402.
- ^ Nau, R; Blei, C; Eiffert, H (17 June 2020). "Intrathecal Antibacterial and Antifungal Therapies". Clinical Microbiology Reviews. 33 (3). doi:10.1128/CMR.00190-19. PMC 7194852. PMID 32349999.
- ^ Grossman SA, Finklestein DM, Ruckdeschel JC, et al. (March 1993). "Randomized prospective comparison of intraventricular methotrexate and thiotepa with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group". Journal of Clinical Oncology. 11 (3): 561–9. doi:10.1200/jco.1993.11.3.561. PMID 8445432.
- ^ Schulmeister L (September 2004). "Preventing vincristine sulfate medication errors". Oncology Nursing Forum. 31 (5): E90–8. doi:10.1188/04.ONF.E90-E98. PMID 15378106.
- ^ Qweider M, Gilsbach JM, Rohde V (March 2007). "Inadvertent intrathecal vincristine administration: a neurosurgical emergency. Case report". Journal of Neurosurgery. Spine. 6 (3): 280–3. doi:10.3171/spi.2007.6.3.280. PMID 17355029.
- ^ International Medication Safety Network (2019), IMSN Global Targeted Medication Safety Best Practices, retrieved 2020-03-11.
- ^ Li, Man; Chen, Hong; Zhu, Mingxin (2022-12-13). "Mesenchymal stem cells for regenerative medicine in central nervous system". Frontiers in Neuroscience. 16. doi:10.3389/fnins.2022.1068114. ISSN 1662-453X. PMC 9793714. PMID 36583105.
- ^ Carrillo-Galvez, Ana Belén; Cobo, Marién; Cuevas-Ocaña, Sara; Gutiérrez-Guerrero, Alejandra; Sánchez-Gilabert, Almudena; Bongarzone, Pierpaolo; García-Pérez, Angélica; Muñoz, Pilar; Benabdellah, Karim; Toscano, Miguel G.; Martín, Francisco; Anderson, Per (2015-01-01). "Mesenchymal Stromal Cells Express GARP/LRRC32 on Their Surface: Effects on Their Biology and Immunomodulatory Capacity". Stem Cells. 33 (1): 183–195. doi:10.1002/stem.1821. ISSN 1066-5099. PMC 4309416. PMID 25182959.
- ^ Vaquero, Jesús; Zurita, Mercedes; Rico, Miguel A.; Aguayo, Concepcion; Bonilla, Celia; Marin, Esperanza; Tapiador, Noemi; Sevilla, Marta; Vazquez, David; Carballido, Joaquin; Fernandez, Cecilia; Rodriguez-Boto, Gregorio; Ovejero, Mercedes; Vaquero, Jesús; Zurita, Mercedes (2018-06-01). "Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline". Cytotherapy. 20 (6): 806–819. doi:10.1016/j.jcyt.2018.03.032. ISSN 1465-3249. PMID 29853256.




