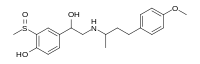
Sulfinalol
 | |
| Names | |
|---|---|
| IUPAC name
4-[1-Hydroxy-2-[4-(4-methoxyphenyl)butan-2-ylamino]ethyl]-2-methylsulfinylphenol
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H27NO4S | |
| Molar mass | 377.50 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfinalol is a beta adrenergic receptor antagonist.[1]
Synthesis
The methyl group on a sulfoxide is sufficiently acidic to substitute for phenolic hydroxyl.
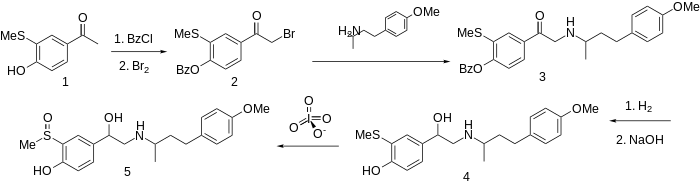
The preparation of this combined α- and β-blocker sulfinalol begins by protection of the phenolic hydroxyl as its benzoate ester. Bromination followed by condensation with 4-(4-methoxyphenyl)butan-2-amine (not PMA) gives the aminoketone 3. Successive catalytic reduction and saponification affords aminoalcohol 4. Oxidation of the sulfide to the sulfoxide with a reagent such as metaperiodate gives sulfinalol (5).
References
- ^ Sybertz, E. J.; Baum, T.; Pula, K. K.; Nelson, S.; Eynon, E.; Sabin, C. (1982). "Studies on the mechanism of the acute antihypertensive and vasodilator actions of several beta-adrenoceptor antagonists". J. Cardiovasc. Pharmacol. 4 (5): 749–58. doi:10.1097/00005344-198209000-00009. PMID 6182405. S2CID 34100646.
- ^ DE 2728641, Philion, Richard Everett, "4-Hydroxyphenylalkanolaminderivate und Verfahren zu deren Herstellung [4-Hydroxyphenylalkanolamine derivatives and processes preparation thereof]", published 1978-01-05, assigned to Sterling Drug Inc.