Neodüüm
Eegenskapen
Algemian
Nööm, Symbool, Numer
Neodüüm, Nd, 60
Seerie
Lantanoid
Skööl, Periode, Blook
La , 6 , f
Klöör, Skak
salwerwitj, lacht güül
CAS-Numer
7440-00-8
Uundial
22 ppm[ 1]
Atomaar [ 2]
Atoommase
144,242(3)[ 3] u
Atoomraadius (bereegent)
185 (206) pm
Kovalent-Raadius
201 pm
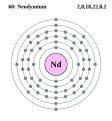
Elektroonen
[Xe ] 4f 4 6s 2
1. Ionisiarang
533,1 kJ /mol
2. Ionisiarang
1040 kJ/mol
3. Ionisiarang
2130 kJ/mol
Füsikaalisk [ 2]
Tustant
fääst
Kristal
hexagonaal
Sachthaid
7,003 g/cm3 (25 °C )[ 4]
Magnetismus
paramagneetisk (Χm −3 )[ 5]
Smoltponkt
1297 K (1024 °C)
Köögponkt
3303 K[ 6] K (3030 °C)
Molaar Rüm
20,59 · 10−6 m3 /mol
Dampwaremk
289 kJ/mol[ 6]
Smoltwaremk
7,1 kJ/mol
Faard faan a tuun
2330 m/s bi 293,15 K
Waremk
190 J/(kg · K)
Elektrisk struumfeerang
1,56 · 106 A/(V · m)
Waremkfeerang
17 W /(m · K)
Cheemisk [ 2]
Oksidatsionstustant
2, 3 , 4
Sür of baasisk
lacht baasisk
Normoolpotentiaal
−2,32 V 3+ + 3 e− → Nd)
Elektronegatiwiteet
1,14 (Pauling-Skala)
Isotoopen
Isotoop
NH
t1/2
Aktiwiteet
Energii (MeV )
Produkt
142 Nd
27,13 %
stabiil
143 Nd
12,18 %
stabiil
144 Nd
23,8 %
2,29 · 1015 a α 1,905 140 Ce
145 Nd
8,3 %
stabiil
146 Nd
17,19 %
stabiil
147 Nd
{syn.}
10,98 d β− 0,896 147 Pm
148 Nd
5,76 %
stabiil
149 Nd
{syn.}
1,728 h β− 1,691 149 Pm
150 Nd
5,64 %
1,1 · 1019 a β− β− 3,367 150 Sm
Muar isotoopen bi List faan isotoopen
NMR -Eegenskapen
Seekerhaid
Miast wurd SI -ianhaiden brükt.
Neodüüm as en cheemisk element mä det ufkörtang Nd an det atoomnumer 60 . Hat hiart tu a lantanoiden . Di nööm komt faan't greks νέος neos ‚nei‘, an δίδυμος didymos ‚twanlang‘ (twanlang faan lantaan ).
Bilen
SIMS -spektrum faan a
isotoopen
Elektroonenskel
Rian neodüüm
Neodüüm glääs
Luke uk diar
Kwelen
↑ Harry H. Binder: Lexikon der chemischen Elemente. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
↑ A taalen för't infobox kem miast faan www.webelements.com (Neodym) .
↑ CIAAW, Standard Atomic Weights Revised 2013 .↑ N. N. Greenwood, A. Earnshaw: Chemie der Elemente. 1. aplaag. VCH, Weinheim 1988, ISBN 3-527-26169-9 , S. 1579.
↑ Robert C. Weast (Hrsg.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , S. E-129 bit E-145. Wäärser diar san uun g/mol an uun cgs -ianhaiden. Heer amgreegent tu SI -wäärs.
↑ 6,0 6,1 Yiming Zhang, Julian R. G. Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. Uun: Journal of Chemical & Engineering Data .doi:10.1021/je1011086 .
↑ 7,0 7,1 Iindraanj tu Neodym, Pulver uun't GESTIS-dootenbeenk faan't IFA , ufrepen di 26. April 2017 (mä JavaScript) .
The article is a derivative under the Creative Commons Attribution-ShareAlike License .
A link to the original article can be found here and attribution parties here
By using this site, you agree to the Terms of Use . Gpedia ® is a registered trademark of the Cyberajah Pty Ltd