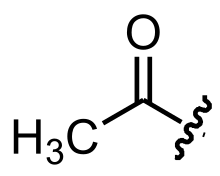
Acetyl
Chemical structure of an acetyl group Skeletal formula of acetyl In organic chemistry , acetyl (ethanoyl), is a functional group , the acyl of acetic acid , with chemical formula -C O C H 3 .
Structure
The acetyl group contains a methyl group bonded to a carbonyl with a lone electron left over. This electron forms a chemical bond to the rest (R ) of the molecule.
Use
The acetyl group is part of some organic compounds such as acetylcholine and acetyl-CoA.
Hydrocarbons (only C and H) Only carbon , hydrogen , oxygen (only C, H and O)
R-O-R
Epoxide Ether
Peroxy
Acetal
Alkoxy
Dioxirane
Ethylenedioxy
Hydroxy
Methylenedioxy carbonyl carboxy
Only one (one element,
Other
Sulfonamide Isothiocyanate
Phosphoramides
Sulfenyl chloride
Thiocyanate
The article is a derivative under the Creative Commons Attribution-ShareAlike License .
A link to the original article can be found here and attribution parties here
By using this site, you agree to the Terms of Use . Gpedia ® is a registered trademark of the Cyberajah Pty Ltd