硫酸铀(IV)
| 硫酸铀(IV) | ||
|---|---|---|
|
| ||
| 英文名 | Uranium(IV) sulfate | |
| 别名 | 硫酸铀、二硫酸铀 | |
| 识别 | ||
| CAS号 | 14355-39-6(无水) 13470-23-0(四水) 19086-22-7(八水) | |
| ChemSpider | 11383849 | |
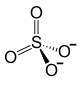
| SMILES |
| |
| InChI |
| |
| InChIKey | SMWCBVIJCHHBAU-NUQVWONBAD | |
| 性质 | ||
| 化学式 | U(SO4)2 | |
| 摩尔质量 | 430.15(无水) 502.22(四水) g·mol⁻¹ | |
| 密度 | 3.6 g·cm-3(四水)[1] | |
| 相关物质 | ||
| 其他阴离子 | 四氯化铀 | |
| 其他阳离子 | 硫酸钕 硫酸钍 | |
| 相关化学品 | 二氧化铀 硫酸铀酰 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
硫酸铀(IV)是一种水溶性铀化合物,化学式为U(SO4)2,高毒。
制备
硫酸铀酰溶液经过光化学还原可以得到硫酸铀(IV),以乙醇为还原剂,光化学还原可以在日光下进行。硫酸铀(IV)在溶液中结晶,或者加入过量的乙醇使之沉淀。硫酸铀有多种水合物。这种光化学反应在热力学和动力学上都是有利的。
- UO2SO4 + 2H2SO4 → U(SO4)2 + 4H2O
相关物种
UIV-SO4可以形成离子及配合物,如USO42+[2]、[U(SO4)5(H2O)]6−[3]等。
参考文献
- ^ 1.0 1.1 Georg Brauer (Hrsg.): Handbuch der Präparativen Anorganischen Chemie. 3., umgearb. Auflage. Band II. Enke, Stuttgart 1978, ISBN 3-432-87813-3, S. 1245.
- ^ Day Jr R A, Wilhite R N, Hamilton F D. Stability of complexes of uranium (IV) with chloride, sulfate and thiocyanate[J]. Journal of the American Chemical Society, 1955, 77(12): 3180-3182. DOI: 10.1021/ja01617a004
- ^ Hennig C, Kraus W, Emmerling F, et al. Coordination of a uranium (IV) sulfate monomer in an aqueous solution and in the solid state[J]. Inorganic chemistry, 2008, 47(5): 1634-1638. DOI: 10.1021/ic701880h
拓展阅读
- Perez, F. M.; Gil, J. M.; Gil, F. J. M. Preparation, infrared and visible spectra of Sulfate Complexes of Uranium(IV). Zeitschrift für anorganische und allgemeine Chemie. 1980, 462: 231. doi:10.1002/zaac.19804620127.
- Merkel, B.; Hasche-Berger, A. (2008). Uranium, Mining and Hydrogeology. Springer. ISBN 978-3-540-87745-5
- Hennig, C.; Schmeide, K.; Brendler, V.; Moll, H.; Tsushima, S.; Scheinost, A.C. (2007). “EXAFS Investigation of U(VI), U(IV), and Th(IV) Sulfato Complexes in Aqueous Solution”. Inorganic Chemistry 46 (15): 5882–5892.
- Cardenas, E.; Watson, D.; Gu, B.; Ginder-Vogel, M.; Kitanidis, P.K.; Jardin, P.M.; Wu, W.; Leigh, M.B.; Carley, J.; Carroll, S.; Gentry, T.; Luoe, J.; Zhou, J.; Criddle, C.S.; Marsh, T.L.; Tiedje, J.M. (2010). “Significant Association between Sulfate-Reducing Bacteria and Uranium-Reducing Microbial Communities as Revealed by a Combined Massively Parallel Sequencing-Indicator Species Approach”. Applied and Environmental Microbiology 76 (20): 6778-6786.
- Converse, B.J.; Wua, T.; Findlay, R.H.; Roden, E.E. (2013). “U(VI) Reduction in Sulfate-Reducing Subsurface Sediments Amended with Ethanol or Acetate”. Applied and Environmental Microbiology 79 (13): 4173-4177.
- Day, R. A.; Wilhite, R. N.; Hamilton, F.R. (1955). “Stability of Complexes of Uranium(IV) with Chloride, Sulfate and Thiocyanate”. Journal of the American Chemical Society 77 (12):3180-3182.
- Hennig, C.; Kraus, W.; Emmerling, F.; Ikeda, A.;. Scheinost, A.C. (2008). “Coordination of a Uranium(IV) Sulfate Monomer in an Aqueous Solution and in the Solid State”. Inorganic Chemistry 47 (5): 1634-1638.
- Plášil, J.; Fejfarová, K.; Novák, M.; Dušek, M.; Škoda, R.; Hloušek, J.; Čejka, J.; Majzlan, J.; Sejkora, J.; Machovič, V.; Talla, D. (2011). “Běhounekite, U(SO4)2(H2O)4, from Jáchymov (St Joachimsthal), Czech Republic: the first natural U4+ sulphate”. Mineralogical Magazine 75 (6): 2739-2753.
- Mudd, G.M. (2001). “Critical Review of Acid in situ leach uranium mining: 1. USA and Australia”. Environmental Geology 41 (3-4): 390-403.
- Závodská, L.; Kosorínová, E.; Scerbáková, L.; Lesny, J. (2008). “Environmental Chemistry of Uranium”. HV ISSN 1418-7108.
| ||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

![{\displaystyle \mathrm {\ \!\ {\Biggr ]}_{2} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)