Indium(I) iodide
| |
| Names | |
|---|---|
| IUPAC name
iodoindium
| |
| Other names
Indium monoiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.034.301 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| IIn | |
| Molar mass | 241.722 g·mol−1 |
| Appearance | red-brown solid |
| Density | 5.32 g/cm3 |
| Melting point | 365 °C (689 °F; 638 K) |
| insoluble | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H315, H319, H334, H335 | |
| P301, P302, P305, P312, P330, P338, P351, P352 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Indium monoiodide is a binary inorganic compound of indium metal and iodine with the chemical formula InI.[1][2]
Preparation
Indium(I) iodide can be obtained by reacting indium with iodine or indium(III) iodide in vacuum at 300 °C to 400 °C or with mercury(II) iodide at 350 °C.[3]
- 2In + I2 → 2InI[4]
- 2In + InI3 → 3InI
- 2In + HgI2 → 2InI + Hg
Physical properties
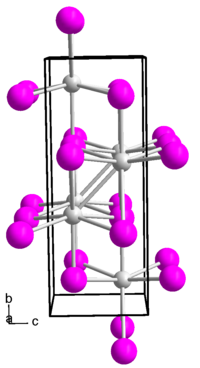
Indium(I) iodide forms a brown-red diamagnetic solid. Its melt is black. The compound has an orthorhombic crystal structure in the space group Cmcm (space group no. 63) with the lattice parameters a = 475 pm, b = 1276 pm, c = 491 pm.[5]
Chemical properties
Decomposes slowly with hot water:
- 2InI + H2O → InOH + HI
Reacts with water in the presence of oxygen:[6]
- 2InI + O + 3H2O → 2In(OH)2 + 2HI
References
- ^ "Indium(I) Iodide". American Elements. Retrieved 29 March 2024.
- ^ "Indium(I) iodide". Sigma Aldrich. Retrieved 29 March 2024.
- ^ Gasanov, A. A.; Lobachev, E. A.; Kuznetsov, S. V.; Fedorov, P. P. (1 November 2015). "Indium monoiodide: Preparation and deep purification". Russian Journal of Inorganic Chemistry. 60 (11): 1333–1336. doi:10.1134/S0036023615110066. ISSN 1531-8613. Retrieved 29 March 2024.
- ^ Rieke, Reuben D. (30 November 2016). Chemical Synthesis Using Highly Reactive Metals. John Wiley & Sons. p. 242. ISBN 978-1-118-92914-8. Retrieved 29 March 2024.
- ^ Fedorov, P P; Popov, A I; Simoneaux, R L (31 March 2017). "Indium iodides". Russian Chemical Reviews. 86 (3): 240–268. doi:10.1070/RCR4609.
- ^ Satya, Prakash (2013). Advanced Chemistry of Rare Elements. S. Chand Publishing. p. 244. ISBN 978-81-219-4254-6. Retrieved 29 March 2024.