Mearnsetin
Mearnsetin
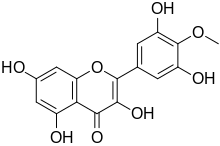 Chemical structure of mearnsetin Chemical structure of mearnsetin
|
| Names
|
| IUPAC name
3,3′,5,5′,7-Pentahydroxy-4′-methoxyflavone
|
Systematic IUPAC name
2-(3,5-Dihydroxy-4-methoxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one |
| Other names
4'-Methylmyricetin
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
|
|
|
| UNII
|
|
|
|
|
InChI=1S/C16H12O8/c1-23-16-9(19)2-6(3-10(16)20)15-14(22)13(21)12-8(18)4-7(17)5-11(12)24-15/h2-5,17-20,22H,1H3 Key: HKEQVXVLTOSXLQ-UHFFFAOYSA-N InChI=1/C16H12O8/c1-23-16-9(19)2-6(3-10(16)20)15-14(22)13(21)12-8(18)4-7(17)5-11(12)24-15/h2-5,17-20,22H,1H3 Key: HKEQVXVLTOSXLQ-UHFFFAOYAX
|
COC1=C(C=C(C=C1O)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O
|
| Properties
|
|
|
C16H12O8
|
| Molar mass
|
332.264 g·mol−1
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Chemical compound
Mearnsetin is an O-methylated flavonol. It can be found in Eucalyptus globulus and in Elaeocarpus lanceofolius.[1] The compound has antioxidative properties.[2]
Mearnsetin 3,7-dirhamnoside can be found in the fern Asplenium antiquum.[3]
References
- ^ Ray, A.B.; Dutta, S.C.; Dasgupta, S. (1976). "Flavonoids of Elaeocarpus lanceofolius". Phytochemistry. 15 (11): 1797–1798. Bibcode:1976PChem..15.1797R. doi:10.1016/S0031-9422(00)97498-3.
- ^ Sadasivam, K.; Kumaresan, R. (2011). "Antioxidant behavior of mearnsetin and myricetin flavonoid compounds--a DFT study". Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 79 (1): 282–93. Bibcode:2011AcSpA..79..282S. doi:10.1016/j.saa.2011.02.042. PMID 21420896.
- ^ Mizuno, Mizuo; Kyotani, Yosuke; Iinuma, Munekazu; Tanaka, Toshiyuki; Kojima, Hiroyuki; Iwatsuki, Kunio (1991). "Mearnsetin 3,7-dirhamnoside from Asplenium antiquum". Phytochemistry. 30 (8): 2817–2818. Bibcode:1991PChem..30.2817M. doi:10.1016/0031-9422(91)85158-V.
Flavonols and their conjugates |
|---|
| Backbone | |
|---|
| Flavonols | | Aglycones | |
|---|
| Conjugates | | Glycosides of herbacetin | |
|---|
| Glycosides of kaempferol |
- Afzelin (Kaempferol 3-rhamnoside)
- Astragalin (kaempferol 3-O-glucoside)
- Kaempferitrin (kaempferol 3,7-dirhamnoside)
- Juglanin (Kaempferol 3-O-arabinoside)
- Kaempferol 3-alpha-L-arabinopyranoside
- Kaempferol 3-alpha-D-arabinopyranoside
- Kaempferol 7-alpha-L-arabinoside
- Kaempferol 7-O-glucoside
- Kaempferol 3-lathyroside
- Kaempferol 4'-rhamnoside
- Kaempferol 5-rhamnoside
- Kaempferol 7-rhamnoside
- Kaempferol 7-O-alpha-L-rhamnofuranoside
- Kaempferol 3-xyloside
- Kaempferol 7-xyloside
- Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside)
- Kaempferol 3-O-rutinoside
- Sophoraflavonoloside (Kaempferol 3-O-sophoroside)
- Trifolin (Kaempferol 3-O-beta-D-galactoside)
|
|---|
| Glycosides of myricetin | |
|---|
| Conjugates of quercetin | |
|---|
|
|---|
|
|---|
| O-Methylated flavonols | | Aglycones | |
|---|
| Glycosides | | of isorhamnetin |
- Narcissin (Isorhamnetin 3-O-rutinoside)
- Isorhamnetin 3-O-glucoside
- Tamarixetin 7-rutinoside
|
|---|
| other |
- Azalein (Azaleatin 3-O-α-L-rhamnoside)
- Centaurein (Centaureidin 7-O-glucoside)
- Eupalin (Eupalitin 3-0-rhamnoside)
- Eupatolin (Eupatolitin 3-O-rhamnoside)
- Jacein (Jaceidin 7-O-glucoside)
- Patulitrin (Patuletin 7-O-glucoside
- Xanthorhamnin (Rhamnetin glycoside)
|
|---|
|
|---|
|
|---|
| Derivative flavonols | | Aglycones |
- Noricaritin
- Dihydronoricaritin
|
|---|
| Glycosides | |
|---|
|
|---|
| Pyranoflavonols | |
|---|
| Furanoflavonols | |
|---|
| Semisynthetic | |
|---|
