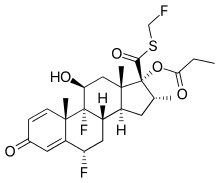
丙酸氟替卡松
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | Flovent, Flixotide, Flonase, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695002 |
| 核准狀況 | |
| 懷孕分級 | |
| 给药途径 | 鼻腔给药,[2] 吸入劑型,[3] 外用藥物[4] |
| 藥物類別 | 類固醇及其衍生物 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 | |
| 藥物動力學數據 | |
| 生物利用度 | 0.51% (鼻內劑) |
| 血漿蛋白結合率 | 91% |
| 药物代谢 | Intranasal 肝臟 (透過CYP3A4) |
| 生物半衰期 | 10 小時 |
| 排泄途徑 | 腎 |
| 识别信息 | |
| |
| CAS号 | 80474-14-2 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.129.097 |
| 化学信息 | |
| 化学式 | C25H31F3O5S |
| 摩尔质量 | 500.57 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
丙酸氟替卡松(INN:Fluticasone propionate)又名氟替卡松丙酸酯。它是一种类固醇药物[8]。吸入劑型用于长期治疗哮喘和慢性阻塞性肺病[8]。鼻噴劑型用于治疗花粉症鼻炎和鼻息肉[9][10] In 2022, fluticasone was the 25th most commonly prescribed medication in the United States, with more than 22 million prescriptions.[11][12]。也可治疗口腔溃疡[13]。
吸入劑的常见副作用包括上呼吸道感染、鼻竇炎、鹅口疮和咳嗽[8]。鼻用劑型的常见副作用包括流鼻血和喉咙痛[9]。主要作用可以减少炎症反應[8]。
丙酸氟替卡松于 1980 年获得专利,于 1990 年取得醫療使用許可[14]。已有學名药流通於市[10]。2018 年,它是美国最常用的处方药第十六名,共開立超过 3千4百万张处方[15] [16]。
参考文獻
- ^ Fluticasone Use During Pregnancy. Drugs.com. 9 January 2019 [31 January 2020]. (原始内容存档于26 March 2019).
- ^ Flonase Allergy Relief- fluticasone propionate spray, metered. DailyMed. 30 May 2019 [31 January 2020]. (原始内容存档于2 December 2020).
- ^ Flovent Diskus- fluticasone propionate powder, metered. DailyMed. 7 January 2019 [31 January 2020]. (原始内容存档于7 December 2019).
- ^ 4.0 4.1 Cutivate- fluticasone propionate lotion. DailyMed. 8 August 2018 [19 February 2022]. (原始内容存档于20 February 2022).
- ^ Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017. Therapeutic Goods Administration (TGA). 21 June 2022 [30 March 2024].
- ^ Respiratory health. Health Canada. 9 May 2018 [13 April 2024].
- ^ Flixonase Aqueous Nasal Spray - Summary of Product Characteristics (SmPC). (emc). 25 October 2019 [31 January 2020]. (原始内容存档于31 January 2020).
- ^ 8.0 8.1 8.2 8.3 Fluticasone Propionate Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [27 February 2019]. (原始内容存档于28 February 2019).
- ^ 9.0 9.1 Fluticasone Propionate eent Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [27 February 2019]. (原始内容存档于28 February 2019).
- ^ 10.0 10.1 British national formulary : BNF 76 76. Pharmaceutical Press. 2018: 262, 1172. ISBN 9780857113382.
- ^ The Top 300 of 2022. clincalc.com. [2024-08-19].
- ^ Fluticasone - Drug Usage Statistics. ClinCalc. [14 January 2024].
- ^ Flixonase aqueous spray (PDF). Sheffield Teaching Hospitals. June 2018 [31 January 2020]. (原始内容存档 (PDF)于25 September 2019).
- ^ Fischer, János; Ganellin, C. Robin. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 487 [28 February 2019]. ISBN 9783527607495. (原始内容存档于28 February 2019).
- ^ The Top 300 of 2021. ClinCalc. [18 February 2021]. (原始内容存档于12 February 2021).
- ^ Fluticasone - Drug Usage Statistics. ClinCalc. [18 February 2021]. (原始内容存档于12 April 2020).
外部連結
 维基共享资源上的相關多媒體資源:丙酸氟替卡松
维基共享资源上的相關多媒體資源:丙酸氟替卡松- Fluticasone Topical. MedlinePlus.