25I-NBOMe
| |||
| (IUPAC) ime | |||
|---|---|---|---|
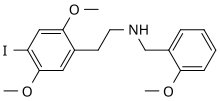
| 2-(4-jodo-2,5-dimetoksifenil)-N-[(2-metoksifenil)metil]etanamin | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 919797-19-6 1043868-97-8 (hidrohlorid) | ||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 10251906 | ||
| ChemSpider[3] | 8427392 | ||
| Hemijski podaci | |||
| Formula | C18H22INO3 | ||
| Mol. masa | 427,2784 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
| Način primene | Sublingualno, potkožno, intravenozno, intramuskularno, rektalno | ||
25I-NBOMe (2C-I-NBOMe) je derivat fenetilaminskog psihodelika 2C-I.[4][5]
25I-NBOMe deluje kao visoko potentan pun agonist ljudskog 5-HT2A receptora,[6][7] sa Ki od 0,044 nM, te je šesnaest puta potentniji od 2C-I liganda. Radio obeležena forma 25I-NBOMe se može koristiti za mapiranje distribucije 5-HT2A receptora u mozgu.[8] In vitro testovi su pokazali da ovo jedinjenje deluje kao agonist, ali rezultati životinjskih studija nisu objavljeni. Dok N-benzilni derivati 2C-I znatno povećavaju potentnost, N-benzilni derivati 2,5-dimetoksi-4-jodoamfetamina su neaktivni.[9]
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ „Ralf Heim PhD. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts. (German)”. Diss.fu-berlin.de. 28. 2. 2010.. Pristupljeno 8. 8. 2012.
- ↑ „Michael Robert Braden PhD. Towards a biophysical understanding of hallucinogen action. Purdue University 2007”. Proquest.umi.com. Pristupljeno 8. 8. 2012.
- ↑ Ettrup, A. et al. (2010). „Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers”. European Journal of Nuclear Medicine and Molecular Imaging 38 (4): 681–693. DOI:10.1007/s00259-010-1686-8. PMID 21174090.
- ↑ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). „Theoretical studies on the interaction of partial agonists with the 5-HT(2A) receptor”. Journal of Computer-aided Molecular Design 25 (1): 51–66. DOI:10.1007/s10822-010-9400-2. PMID 21088982.
- ↑ Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH (June 2008). „High Specific Activity Tritium-Labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): A High Affinity 5-HT2A Receptor-Selective Agonist Radioligand”. Bioorganic & Medicinal Chemistry 16 (11): 6116–23. DOI:10.1016/j.bmc.2008.04.050. PMC 2719953. PMID 18468904.
- ↑ Braden, MR; Parrish, JC; Naylor, JC; Nichols, DE (2006). „Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists”. Molecular Pharmacology 70 (6): 1956–64. DOI:10.1124/mol.106.028720. PMID 17000863.